Overview: Metal Salt Solutions
Metal Salt Solutions
Metal Salt Solution are ionic salt solutions that contain metal elements as positively charged cations, and non-metal as negatively charged anions when dissolved in solution. These compounds can regulate ionic concentrations of the solutions they are dissolved in, affecting the pH, osmolality, conductivity, refractive index, and many other physical and biological attributes of final solutions. They are also key performers in regulating osmotic pressure when combined with other salt types in Balanced Salt Solutions, and readily dissolve in the presence of water as a solvent.
Ionic salts used to create Metal Salt Solution like Sodium Chloride solution, Calcium Chloride Solution, Potassium Chloride solution, or Magnesium Sulfate solution help to mimic the internal ionic concentrations of cell types of interest, ensuring the isotonic environment of cell culture experiments.
Ionic Dissociation
When ionic compounds such as Metal Salt Solutions are combined with polar solvents such as water, ionic dissociation occurs, or the separation of cations and anions of ionic compounds. This process allows the ionic salt to completely dissolve within the solvent, ultimately increasing the concentration of solute dissolved, and ions in solution. At the molecular level, the metal cation, for example the sodium atom in sodium chloride, is attracted to the partially negative oxygen end of the water molecules as it enters solution, while the negative chlorine anion is attracted to the partially positive end of the water molecules. This allows these polar water molecules to completely pull apart the ionic interactions between the cations and anions of metal salts, resulting in a completely saturated solution. When dissolved, these charged ions carry electrical charges, enhancing the overall conductivity of the solution.
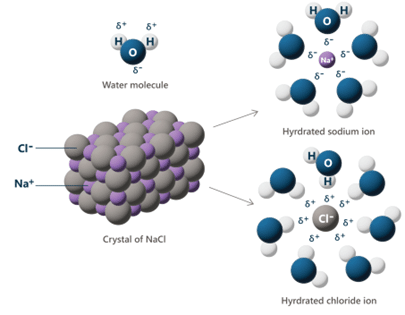
Image 1: The dissociation of crystalline Sodium Chloride (NaCl) when introduced with water (H2O). The polar nature of water helps to break the ionic bonds of sodium chloride based on charge, as the partially positive (δ+) hydrogen atoms are attracted to negatively charged Chloride ions (Cl-), and the partially negative (δ-) oxygen atoms are attracted to positive Sodium ions (Na+). This polar interaction allows metal salts to become completely saturated in water..
Ionic dissociation is a key step in many chemical reaction types, including redox reactions, and acid-base reactions, as this process affects the formation and stability of ions, transfer of electrons, and overall stability of substances. These reaction types are often common in cellular processes and can be observed below.
- Redox Reactions: Redox (Red=reduced, or gaining electrons and Ox=Oxidized, or losing electrons) is a reaction in which one or more atoms gain or lose electrons. Ionic dissociation is important for redox reactions because it determines the oxidation state of each element and the potential for electron transfer. For example, when iron (Fe) reacts with oxygen (O2 to form iron oxide (Fe2O3), the iron atoms lose electrons and become Fe3+ ions, while the oxygen atoms gain electrons and become O2- Prior to this reaction, ionic dissociation must occur in solution [1].
- Acid-Base Reactions: These are reactions in which an acid or a base reacts with another substance to produce salt and water. Ionic dissociation is important for acid-base reactions because it determines the concentration of the reactants and pH of the solution. For example, when hydrochloric acid (HCl) dissolves in water to form H+ ions and Cl- ions, the equation for this reaction is: HCl(aq) à H+(aq) + Cl-(aq). If this reaction occurs in a metal solution such as magnesium sulfate, the dissociated magnesium sulfate would react with the dissociated HCl to form magnesium chloride and water, ultimately minimizing the pH increase of the system.
Metal salt solutions are specifically versatile tools, they can also:
- Form complexes with organic molecules to increase or decrease solubilities and molecular mobility.
- Act as reducing agents to prevent toxicity of substances. This is especially useful when experimenting with biologics including viral vectors.
- React to form metal complexes promoting the precipitation of compounds out of solution [2]
- Provide scientists with a variety of use types depending upon their applications and can be formulated specific to unique studies.
Metal Salt Solutions at Boston BioProducts
Every Metal Salt Solution is unique to the cell type used and the experimental application. Select the appropriate metal salt solutions from the catalog or design your optimal formulation with custom manufacturing options at Boston BioProducts.
Build a Custom Reagent Quickly
References:
-
- Grundl, T. J., Haderlein, S., Nurmi, J. T., & Tratnyek, P. G. (2011). Introduction to aquatic redox chemistry. In P. G. Tratnyek, T. J. Grundl, & S. B. Haderlein (Eds.), Aquatic Redox Chemistry (pp. 1-14). ACS Symposium Series (ACS Publications)
- Martell, A. E., & Hancock, R. D. (2013). Introductory overview. In Metal Complexes in Aqueous Solutions (pp. 1-14). Metal Complexes in Aqueous Solutions | SpringerLink
- Burgess, J. (1999). Introductory overview. In Ions in Solution: Basic Principles of Chemical Interactions (pp. 1-14). Ions in Solution: Basic Principles of Chemical Interactions - J Burgess - Google Books
