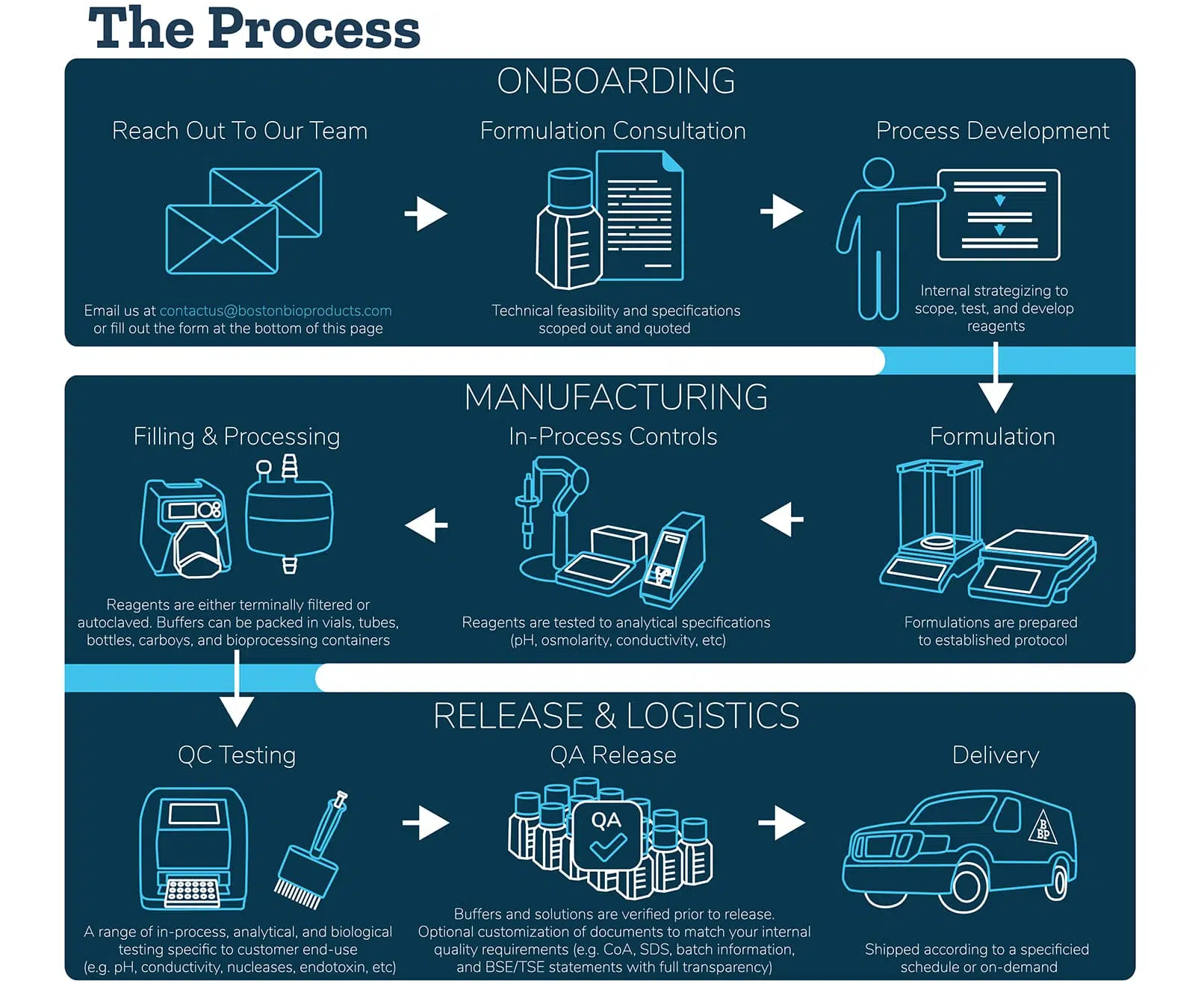
What you can customize:
Our team has the knowledge and capabilities in custom buffer manufacturing to develop virtually any buffer for any application. On top of that, expect a fast delivery. With our optimized delivery process, you’ll receive your custom buffers and cell culture media in days to weeks, not months.
Formulation & Specs
Specify the recipe, concentration, pH, and protocol to receive a buffer fit for your specific application in research and drug development.
Raw Material Selection
Provide us with raw material details or use those sourced from our verified vendor network. We support research-grade, compendial, and multi-compendial raw materials.
Filling & Processing
Lets optimize your method.. We can incorporate 0.2 micron filtration, terminal sterilization, and direct fill into the manufacturing protocol.
Quality & Performance Tests
Verify physical characteristics, physiological properties, and performance. We support 10+ analytical and biological tests such as endotoxin, nucleases (DNase and RNase), and microbial screening.
Volume & Containers
Get low minimum quantities of mililiters up to hundreds of liters and have it packaged in your choice of vials, bottles, carboys, or bioprocessing containers.
On-demand Quality Documentation
Customize the documentation to match your internal QMS. We provide CoA, SDS, batch information, and BSE/TSE statements with full transparency.
Why partner with Boston BioProducts
Flexibility
Buffers are our primary focus, whereas other companies sell buffers as an additional, tertiary product line.
We are dedicated to meeting our customers’ wide-ranging, ever-changing needs. Our services are customizable for diverse applications in research, drug development, kitting, and diagnostics. You also have your choice of delivery schedule, storage options, and more.
Scalability
As we go through the process of optimizing formulations, we always consider its scalability. We know what scales well and performs effectively.
This helps our customers avoid time-consuming buffer re-optimization as they move from mLs to Ls to gallons. Our 115,000 ft2 facility is ISO 13485:2016 certified and designed to support RUO to GMP scale-up.
Reproducibility
You can rest assured that our batch-to-batch consistency is high. We use proven formulations that follow exact production procedures to create the same product each time it is manufactured.
A thorough onboarding process for new projects keeps our technicians up-to-date and organized. We catalog and record each step for every batch of material. Our quality assurance processes ensure you receive exactly what you expect.
Speed
Product availability is crucial for establishing a smooth workflow. We work diligently to ensure that your scientists are always experiment-ready and your lab is stocked for the next run. Our streamlined process enables a rapid turnaround. Think days to weeks, not months.
You can get on-demand custom buffer manufacturing of repeat orders and use our long-term storage options to keep your material readily available.