Overview: Buffer Solutions for Optimizing pH
Buffers
Biological buffers are stabilizing solutions used to maintain pH and replicate biological environments found in organisms. These compounds consist of weak acids and conjugate strong bases (or vice versa), operating through dynamic equilibria that resist hydrogen ion changes when combined with other acidic or basic substances. For instance, an Acetate Buffer, composed of acetic acid and acetate ions, efficiently maintains pH stability for its related applications within the pH range of 3.8 to 5.8. The equation of an Acetate Buffer can be reviewed as seen below:
1 2 3 4
CH3COOH (aq) + H2O (l) ⇌ CH3COO- (aq) + H3O+ (aq)
Where:
Acetic acid (1), a weak acid, is combined with water (2), resulting in its deprotonation into a strong conjugate base known as an acetate ion (3), and a subsequent hydronium ion (4).
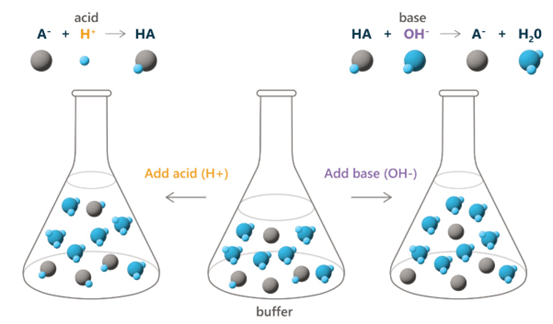
This aqueous solution is effective at regulating the pH of an environment from a pH of 3.6 to 5.6, as the pKa value (the negative logarithm of the dissociation constant) for acetic acid is 4.6. This means that at a pH of 4.6, roughly 50% of all Acetic acid (1) molecules are deprotonated. To effectively maintain the pH of a system, this acetic acid buffer follows a natural phenomenon known as Le Chatelier’s Principle[1], which states that when stress is applied to a system in the form of acidic or basic substances, or a change in concentration, the system will adjust to minimize the effect of the stress. For example, if the hydronium ion (4) concentration were to increase in an acetic acid buffer due to an increase in acidic content added, the reverse of this reaction would occur, and therefore creating more Acetic acid (1) and water (2), subsequently lowering the hydronium ion (4) concentration. This regulates the pH of the system within the given pH range and is performed by biological buffers to regulate experimental environments.
The Importance of pH Regulation
The pH level of experimental or biological environments influences enzymatic activity and cellular processes. Even the slightest change in hydrogen ion concentration can adjust an experiment’s products. Additionally, the buffering capacity, determined by buffer type and concentration, is directly correlated to a buffer’s performance.
The understanding of buffering capacities as well as component pKa values can determine the ideal buffer and can be calculated using their associated equations.
![]() Where:
Where:
- n represents the molar concentration of the acid or base added per liter of buffer
- ΔpH represents the change in pH caused by the addition of acid or base
This formula demonstrates that buffer capacity (β) is determined by the ratio of the change in concentration of the buffer solute to the change in pH. A higher buffer capacity (β) indicates that the buffer solution can effectively resist changes in pH when acids or bases are added, emphasizing the importance of concentration in buffer efficacy. To calculate the pH of a substance, the Henderson-Hasselbach equation[4] can be observed.
![]() Where:
Where:
- pH represents the acidity or alkalinity of the solution.
- pKa is the negative logarithm of the acid dissociation constant (Ka) for the weak acid. It is a measure of the acid's strength; a lower pKa indicates a stronger acid.
- [A-] represents the concentration of the conjugate base (e.g., acetate ion, CH3COO-) in the solution.
- [HA] represents the concentration of the weak acid (e.g., acetic acid, CH3COOH) in the solution.
Find the Ideal Buffer for Optimal pH Maintenance
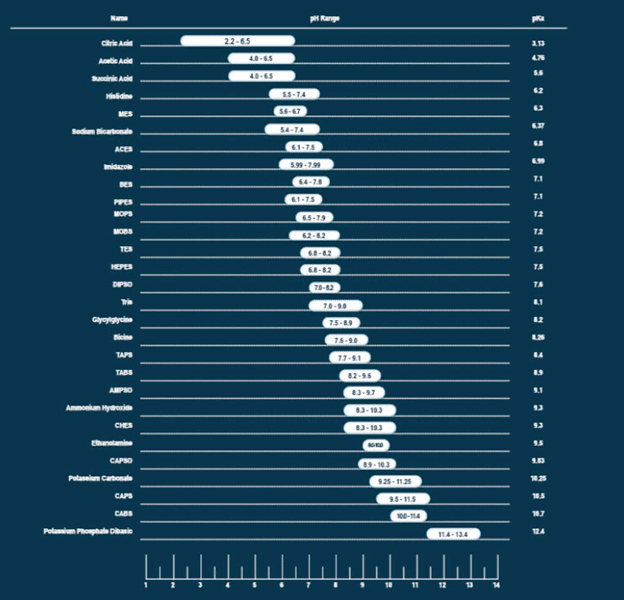
Buffers at Boston BioProducts
Every buffer is unique to its intended application. Experience thousands of different buffer combinations and select the one that matches your exact requirements in terms of pH, concentration, and conductivity by exploring custom manufacturing options at Boston BioProducts, or our catalog buffers.
Build a Custom Reagent Quickly
References:
-
- Peris, M. (2021). Understanding Le Châtelier’s principle fundamentals: five key questions. Chemistry Teacher International, 4(3), 203-205.
- Ferreira, C. M. H., Pinto, I. S. S., Soares, E. V., & Soares, H. M. V. M. (2015). (Un)suitability of the use of pH buffers in biological, biochemical and environmental studies and their interaction with metal ions – a review. RSC Advances, 5(39), 30989-31003.
- Michałowska-Kaczmarczyk, A. M., & Michałowski, T. (2015). Dynamic buffer capacity in acid–base systems. Journal of Solution Chemistry, 44(6), 1256-1266
- Po, H. N., & Senozan, N. M. (2003). The Henderson–Hasselbalch equation: Its history and limitations. Journal of Chemical Education, 80(2), 146-151
- Thornbury, G. A., Shaheen, F., Al-Awadi, N., Neelakanda, P., & Al-Muhtaseb, A. H. (2014). Good’s buffers as a basis for developing self-buffering and biocompatible ionic liquids for biological research.
- Good, N. E., Winget, G. D., Winter, W., Connolly, T. N., Izawa, S., & Singh, R. M. M. (1966). Hydrogen ion buffers for biological research.
